Lipid Nano Particles (LNP)
RNA therapeutics has rapidly developed since a great success of RNA vaccines against SARS-CoV-2 virus. Encapsulation into Lipid NanoParticles (LNPs) has been proven to be one of the most efficient ways for RNA delivery in vivo. The approach uses lipid mixtures with positively charged cationic lipids that interact to negatively charged RNA molecules, protecting them from from enzymatic degradation and providing intracellular penetration. In a joined effort with Abisch-Frenkel RNA Therapeutics Center (Prof. Igor Ulitsky), we established a publically available facility for RNA-LNP production. Our facility integrates equipment required for LNP assembly, post-assembly processing and quality control. It allows production of minimum 2 ml of RNA-LNPs that covers most of the research needs.
NanoAssembler Ignite:

The Ignite system (Precision NanoSystems) is designed to assemble RNA lipid nano particles particles (LNPs), using microfluidic cartriges. Easy-to-use programming provides high reproducibility of the LNP assembly. Either custom lipids formulations or a commercial kit (GenVoy-ILM) can be applied for RNA encapsulations. Disposable NxGen cartriges eliminates risk of cross-contamination.
Zetasizer:

ZetaSizer Ultra (Malvern Panalytical) is the most advanced analyser enabled to measure (1) size of nanoparticles, (2) particle concentration, and (3) Z-potential of nanoparticles. The device enables to analyse particles up to 1 um. Estimation of particle size and Z-potential is a necessary step of LNP quality control, but can also be used in other applications, like analysis of aggregates.

Typical output of mRNA encapsulation by the GenVoy-ILM lipids. ZetaSizer detects a single pick of 120nms.
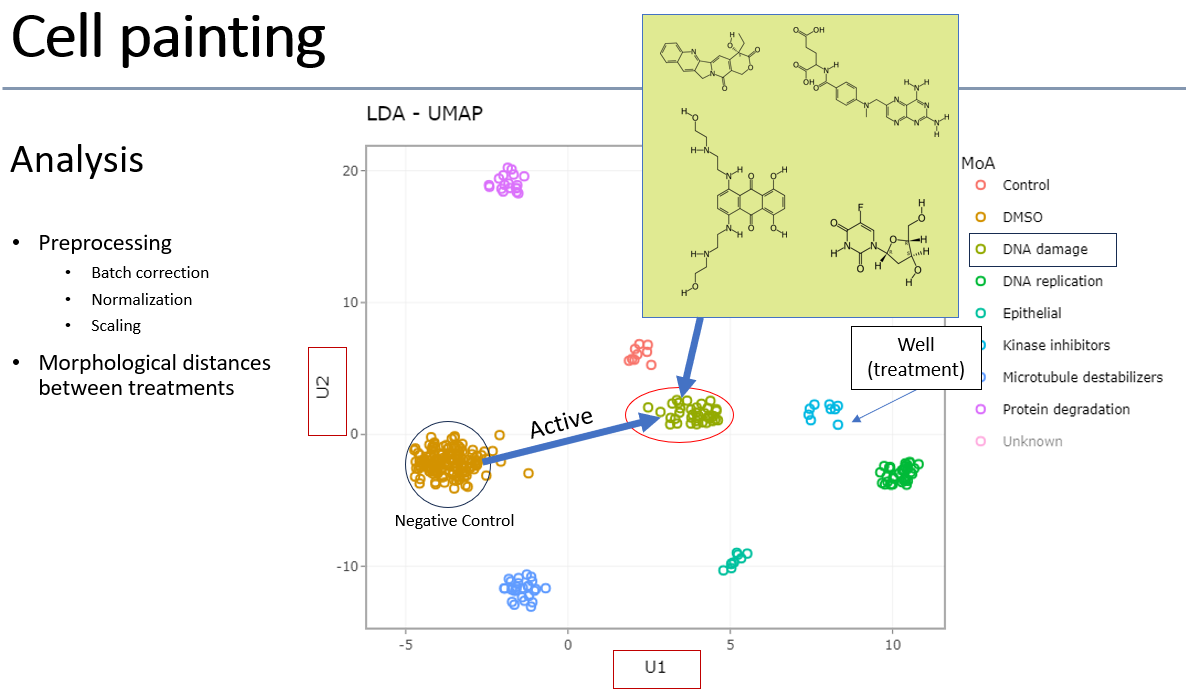
Cell Painting
Cell painting is an assay developed by the Carpenter lab in Broad Institute of Harvard/MIT. Since the original publication (Bray, 2016) it has become a widely-used method to characterize chemical and genomic perturbations of cells using generic organelle dyes, high-content microscopy, image-based feature extraction, and multi-dimensional analysis. The image quality of the cells is important for good results, so commonly U2-OS cells are used in cell painting. Features are extracted from images using the open-source Cellprofiler software. Up to 3000 assays can be executed per run in our lab, with the image analysis and interpretation done by the INCPM Bioinformatics unit.
Example of cell painting analysis:
Compressed screening
A compressed phenotypic screen is a high-throughput experimental approach used in drug discovery or chemical biology, where multiple compounds are tested together in the same well of a multi-well plate. In the following experiment, wells of interest (i.e. showing a significant response) are deconvoluted by repeat of the assay with individual hit compounds in individual wells.
The primary aim of this approach is saving of reagents and time. It can also assess the collective effects of combinations of compounds on a specific phenotype or biological process, i.e. interactions between compounds, such as their additive, synergistic or antagonistic effects.
A recent (unpublished) screening campaign of 42,000 compounds was executed in 4X compression (i.e. 4 compounds per well) with 14 compounds reproducing the response after deconvolution and confirmation.

